
Pricking my finger then measuring the level of ketones in my blood.
April 16, 2024 — I pricked my finger and moved a disposable ketone measuring stick into the newly formed drop of blood. I was checking my "blood ketone" levels. If the result came back higher than 0.8 mmol/L, I would be in a state of "ketosis".
The meter showed "1.0 mmol/L". Success! I was still in ketosis.
It was day 174 since, at wits' end, I started a therapeutic ketogenic diet as a treatment strategy for bipolar disorder.
Before hearing about keto for bipolar I had tried and failed nearly all of the major bipolar treatment strategies.
One, lithium, is often called the "gold standard" bipolar treatment.
I tried lithium. Three times.
I'll admit, lithium did seem to stabilize my energy, but there was a serious downside: the lithium slingshot, I call it.
The side effects and annoyances of taking lithium are constant. Some parts of my brain and body are objectively worse on it. Brain fog. Weight gain. That sort of thing.
So, even if I took lithium correctly for 300 days, on day 301 parts of me would still be voting to stop.
And so, inevitably, at some point parts of me would find an excuse to stop. Then, within weeks, I would enter a state of hypomania or mania. As the lithium tapered out of my system, my brain energy would shoot forward way past the starting point. The lithium slingshot.
I looked at the ketone meter again: 1.0 mmol/L.
I paused. There was something familiar about that number.
Then I remembered. Years ago my doctor said we were aiming for a lithium blood level of 1.0 mmol/L.
To be in therapeutic ketosis I needed ketones to be around one millimole.
To be in therapeutic lithium I needed lithium to be around one millimole.
Both molecules have neuro effects when they are around one millimole.
What are the odds? We can measure chemicals at 100 nanomole levels, 10 nanomole levels, 1 nanomole levels, and at levels below that and everything in between.
Was it just a random coincidence that these 2 different chemicals had overlapping therapeutic windows, or is this a clue to some common mechanism(s)?
Unfortunately, it had been 22 years since high school chemistry class, and I couldn't even remember what a millimole was. This was going to take me some time.
But I had to know the answer. You could even say there was a chance my life depended on it.
So, I dove in. This is the story of coming up with an answer to the question: what are the odds that the therapeutic windows for 2 different chemicals would overlap?
Lithium
Taking lithium was annoying.
I had to swallow pills each day. I am a competitive person, but in a pill swallowing competition I would lose to pretty much anyone.
I suck at swallowing pills. If they are larger than an M&M I'm going to need 3 because I'll gag and cough up the first two.
I don't know why I'm worse than others at swallowing. Maybe it's from that summer on Cape Cod when I was a kid and left the kitchen with my mouth full and almost choked to death on a piece of undercooked bacon. I made it back to the kitchen seconds before I passed out, my dad saw what was happening, gave me a pop, and saved my life. Maybe ever since that day my brain has turned every unchewed thing in my mouth into a piece of undercooked bacon.
Now, if it was just discomfort from swallowing pills, I'm sure I could focus on that and learn.
But it's not just swallowing the lithium. You also have to get the lithium.
I can go to a liquor store and buy enough alcohol to supply a bar for a decade, but god forbid I be allowed to keep a year's supply of a supposedly "gold standard life saving" medicine on hand. Instead I had to go to a pharmacy every month or so, often getting a different variant of lithium pills (extended release; different shapes; different sizes, etc) which I could never predict beforehand, and never being quite sure what I had to pay until I was at the register.
More than that, to keep the lithium prescriptions coming I had to maintain an expensive relationship with a psychiatrist or nurse practitioner. The system tells us the worst thing you can do is stop taking your meds but then is designed to make it as easy as possible to stop taking your meds. As far as I can tell bipolar meds can only be managed successfully long term if you are the type of person as predictable as the moon, and bipolar people decidedly are not.
Of course, there are real safety concerns with lithium. At high enough concentrations it can damage your kidneys and even kill you. So taking lithium came with another annoyance: laboratory blood tests. Measuring lithium in your blood is harder than measuring other things, such as glucose and ketones. (I say this now as if I already knew that, but I knew almost nothing about any of this until I set out to answer that first question). I can tell you from experience at-home blood tests are a 100x better experience than lab tests.
To sum up, not even talking about side effects but for logistical reasons alone, taking lithium was annoying.
Keto
Keto is different.
There are no pills to swallow. There are no prescriptions to perpetually refill. There is no relationship with a licensed provider I have to maintain to keep getting those prescriptions. I don't need to worry about toxicity levels[1]. And I can do the blood tests myself, at home.
The "side effects" from eating healthier whole foods include weight loss, better teeth and better skin.
(I do need to note that there are still many long term unknowns around keto and like anything, if done improperly, there can be serious health consequences[1]. And it is sad to say no to real bagels, pizza and pasta).
But here's the thing about keto for bipolar disorder: it is basically brand new. Although the idea of the therapeutic ketogenic diet has been out for over a hundred years and well studied for epilepsy, how keto could be used for bipolar disorder has just barely been looked at by science. In fact, if it weren't for a few curious pioneers[2] and philanthropists, it would remain barely looked at by science.
Now there's a serious effort underway to test and better understand keto for bipolar. In a few years, we will know a lot more.
Who knows, maybe the expanded science may cause ketones to pass lithium as the new gold standard in bipolar.
Meanwhile, while we wait for the professional scientists to come to confident, big data backed answers, amateur scientists, like yours truly, have gathered in online forums to share knowledge and try and help get to an understanding of keto for bipolar faster.
And so now I return to the one millimole question.
What is a ketone?

Ketones are tiny molecules. The ketone BHB (beta-hydroxybutyrate) accounts for around 70-80% of your ketones. Image Source: NIH
A ketone is small. Very small. The diameter of a ketone is 0.6 nanometers. Have you heard how small an atom is? A ketone is not much bigger. A ketone is only 10-15 atoms (hydrogen, carbon, and oxygen). Ketones are so small, that if you layed 20 million of them end to end, you would only make it across one penny.
There are 3 kinds of ketones. They have long chemical names. BHB (C_4H_8O_3), which makes up about 70-80% of your ketones. AA (C_4H_6O_3), which makes up around 20%. And Acetone (C_3H_6O), which makes up around 2%.
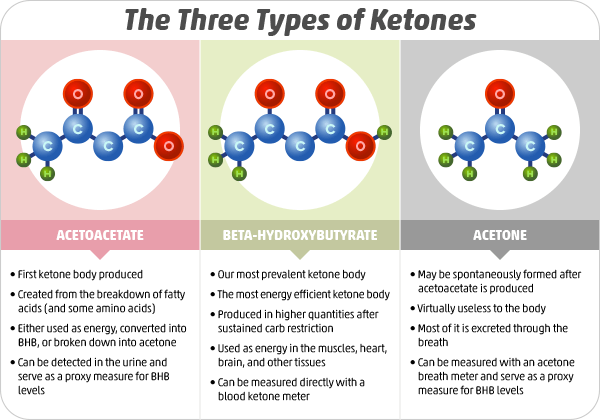
BHB is the one we care most about. It is the one most abundant. When I prick my finger and measure my ketone levels, I'm measuring BHB.
Ketone test strips contain a molecule called β-hydroxybutyrate dehydrogenase. This reacts with the BHB. This reaction is correlated with a change in electrical current, which is measured by the handheld device, and communicated to me as my ketone blood level (a technique called Amperometry).
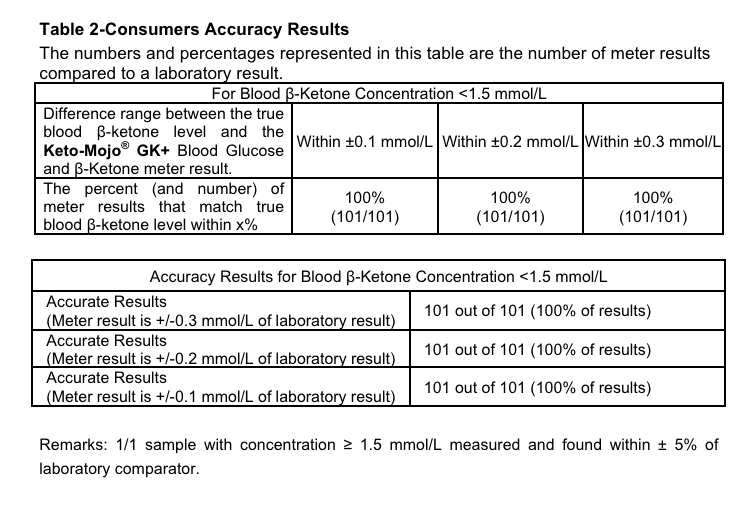
At home finger prick ketone blood tests are highly accurate. Source: Keto Mojo GK+ manual
Somewhere around 90% of ketone production happens in the mitochondria of the liver cells (hepatocytes)[3]. The liver is actually the largest organ inside the human body (after that is the brain, lungs, heart, ...). You can't live without a liver. Maybe that's why they call it the liver?
Ketones are small and water soluable. From the liver, ketones enter the blood plasma (not the blood cells) to reach other destinations in the body. Blood plasma makes up about 55% of the volume of the blood, is mostly water, and distributes important things like ketones throughout the body.
Ketones that are not used by cells for energy production leave the body through urine (60-80%), breath (15-25%), and sweat (<5%). To get in your urine, ketones are filtered from your blood by your kidneys, travel down your ureters, into your bladder, and then travel out into the world, emitting a fruity "ketone" smell.
Sidenote: It is interesting that humans are able to smell ketones. I wonder why we would have evolved an olfactory sensitivity to them. Is it a positive smell, or a warning sign?
For ketones to reach brain cells, they travel in the blood plasma from your liver to your brain, then cross the blood brain barrier (which has mechanisms to permit the entering of ketones), into the Interstitial Fluid of the brain, and from there are taken into the cells, where they are used in the Krebs cycle to make ATP.
Okay. So I can describe a ketone. What about a millimole of ketones?
Turns out, that is a lot of ketones.
One mole is ~ 6 \times 10^{23} . This means that one millimole of ketones is:
602,200,000,000,000,000,000 ketones
Wow! I forgot how big the small world is.
If I was able to pile up all the ketones in my blood, how much would that amount to?
The amount of whole blood (blood cells + blood plasma), varies depending on the size of the person. I am 5'10", 170lbs, which means I have roughly around 6 liters of blood in me.

If you took out all my blood, this is roughly what it would look like.
I will leave the math in the comments, but the final answer is 0.5mL worth of ketones.
This means, if you gathered all the ketones in my blood right now, despite it being an absolutely massive number of ketones, you'd have around a blueberry's worth of ketones.

If you gathered all the ketones circulating in my blood right it would be about the size of a blueberry.
I told you they were tiny.
Of course, the amount of ketones in my blood at any one moment is not the same as the amount of ketones my liver is producing. I mean, the purpose of ketones aren't to circulate in the blood but to be consumed by cells.
We measure ketones in the blood, because that's the easiest place to measure them, but they are in other places too, like your cerebral spinal fluid, sweat, urine, and 💩.
Estimates vary on how many ketones my body would produce in a day while in ketosis, but 100 grams of ketones per day seems to be a decent ballpark, which amounts to around one mole of ketons (1,000 millimoles), which, if we wanted to visualize it, would be about the size of 200 blueberries:

All the ketones made by a person in a day might be around 200 blueberries of ketones.
Alright, so now we have a better sense of what a millimole of ketones is. Let's turn our attention to lithium.
What is lithium?
Lithium carbonate is also a tiny molecule. Image Source: PubChem
There are around four lithium salt compounds in use for medicinal purposes. Lithium Carbonate (Li_2CO_3) is by far the most common, around 95% of the lithium prescribed in the world. Lithium orotate (C_5H_3LiN_2O_4) is the second most popular, accounting for around 3%-4%. Lithium Citrate and Lithium Aspartate are the least used. Lithium Chloride was also used medicinally in the past before a majority opinion arose that it was more toxic than the others.
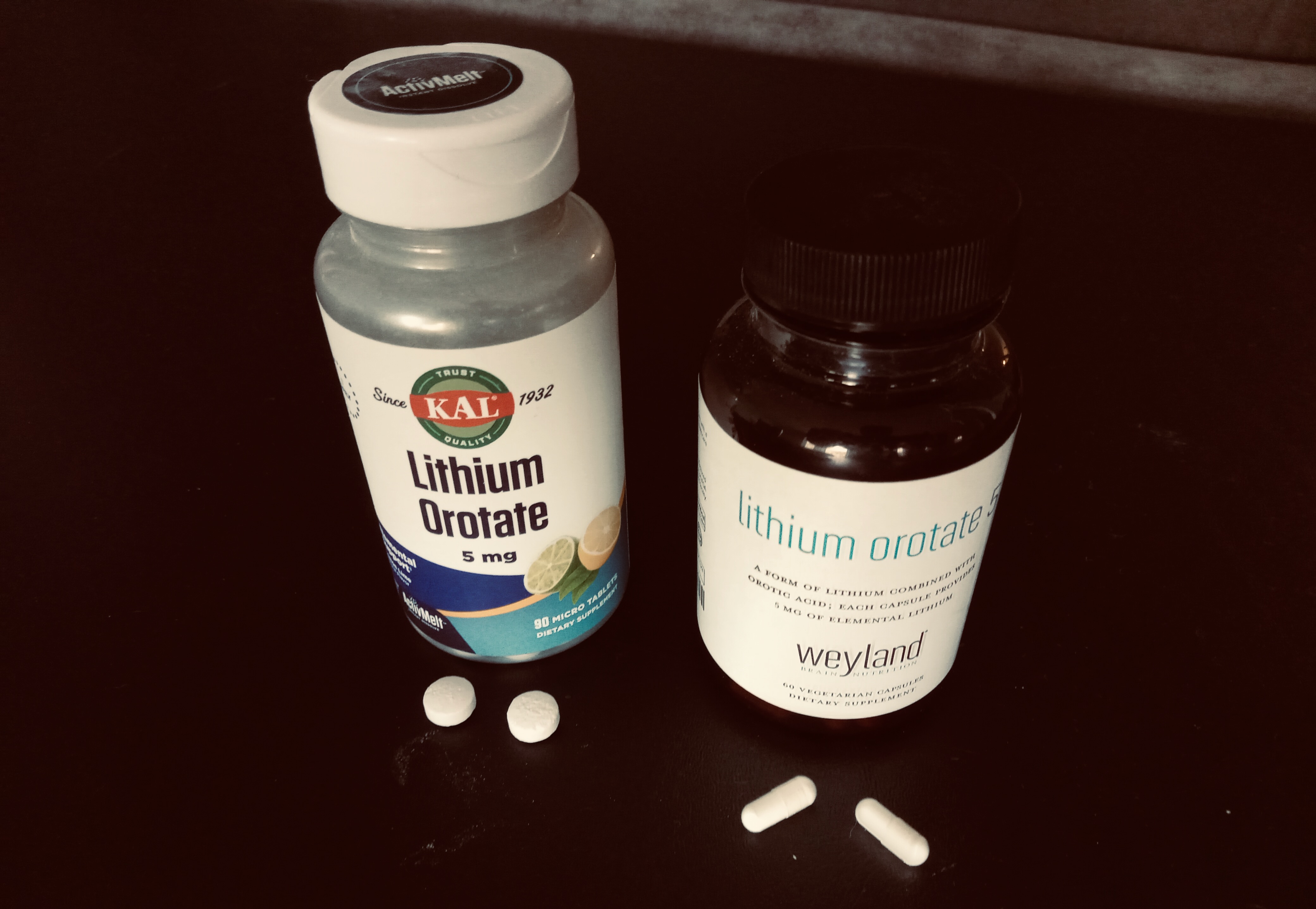
Lithium in pill form. Lithium orotate (shown above), is studied much less than Lithium Carbonate, but one advantage it has is that you can buy it cheaply online, with no prescription. Unfortunately lithium at high levels is toxic and there is no at home lithium testing yet.
Because it is the dominant form, for the rest of this post when I say "lithium" I am referring to Lithium Carbonate.
Just like ketones, lithium is tiny. In fact, according to my calculations, a molecule of lithium is around 2.5x smaller than a ketone molecule.
Unlike ketones, you cannot check your lithium levels at home (though some people are working on that). Instead, to measure lithium levels your blood needs to be processed in a lab using techniques with names like Ion-Selective Electrode, Atomic Absorption Spectroscopy, Inductively Coupled Plasma Mass Spectrometry or Flame Emission Spectroscopy.
I can't really find a great reason why at home lithium tests aren't available. Some people write that it wouldn't be safe, because getting accurate readings is more difficult, but I think multiple at home readings, even if off a bit, would be better than no readings at all. If I had to pick an explanation, it would be that because of diabetes, there's a big market for glucose and ketone tests, but it's relatively rare to take lithium so there isn't a big enough market for a company to build a convenient at home lithium test at this time.
After you swallow your lithium the pills land in your stomach. There, fluids dissolve them. The lithium (Li+) splits from the carbonate. The next destination in your gastrointestinal tract is your small intestines, a place which happens to allow ~100% of the lithium ions to be absorbed into your bloodstream.
Like ketones, lithium crosses the blood brain barrier. Unlike ketones, lithium cross via passive diffusion. Ketones use active transport (well, not all ketones. Acetone can cross passively).
Once lithium is in your brain, it both remains in the extracellular space and also is able to enter neurons.
When a lithium ion enters a cell, it may hang around for quite a bit, but eventually when it leaves it is the same lithium ion. This is different than a ketone molecule, which undergoes metabolic transformations in cells.
Like ketones, your body excretes lithium in your urine and sweat. (Sidenote: Unlike ketones, humans have not evolved a smell sensitivity to lithium).
Another lithium test a lab can do is called an RBC test. This test measures the levels of lithium ions that have entered your cells (red blood cells specifically). To perform this test, your red blood cells are first separated from your blood plasma, then they are lysed (split open) so their contents can be measured. It seems lithium levels in blood plasma spike quickly after ingestion, then decrease quickly, but levels change more slowly in other places, like red blood cells, neurons, and CSF (Cerebrospinal fluid).
This seems to explain why my lithium slingshot did not happen immediately after stopping lithium but in the weeks after. Once you stop taking lithium, the lithium content in your blood runs out fast, but it takes more time for the lithium to leave all of your cells. Then, those cells, which had been working in a lithium rich environment, seem vulnerable to catching a manic fire. The lithium slingshot.
Can we see the amounts of lithium in the brain? Apparently we can, to some degree, using MRS. Ketones too. I looked into being able to take my own MRS pictures at home, but didn't have a place to put one of the machines. Oh yeah, and they cost $500,000!
I also want to note something interesting about lithium blood levels vs ketone levels. We mentioned earlier that when in ketosis your body is constantly producing and consuming ketones, so many that it would amount to over 100 blueberries throughout the day. But lithium is exogenous and you usually take it just once or twice a day, so your body is exposed to just a few blueberries of lithium a day. But the blood levels are similar. So where are all those ketones going? It seems that your cells must be grabbing those ketones from the blood far faster than lithium is grabbed from the blood.
Lithium seems to move more slowly into cells compared to ketones. Perhaps the response time to ketones might be 2x-10x greater than to lithium. Again, this is probably the reason for the delayed lithium slingshot.
Whew. That was a lot to learn.
Now, armed with these basic model of ketones and lithium, I can start returning to the main question of this post.
One millimole: what are the odds?
This whole post began because I noticed that 2 very different treatments for bipolar disorder, a ketogenic diet and lithium, use blood tests to determine if someone is in a therapeutic range, and it just so happens that even though each is testing for the presence of something different (ketones vs lithium), if your level is 1.0 mmol/L then you are in the therapeutic range in both treatments.
Now of course, the ranges are not identical. For lithium, the therapeutic range is generally given as between "0.4 and 1.2 mmol/L". Lithium levels above 1.5 mmol/L are considered toxic and above 2.0 mmol/L can be life-threatening.
The range for nutritional ketosis is usually given as between "0.5 and 3.0 mmol/L". Some say ketosis begins above 0.8mmol/L. Some say nutritional ketosis requires levels above 1.5mmol/L.
Admittedly then my "one millimole" is a slight simplification, as the target therapeutic ranges are not identical, but there is overlap[4].
We can measure a ton of chemicals in the body. Different chemicals have different effects in different concentrations. Should we be surprised at all that two different chemicals both seem to treat the same condition at similar concentrations?
Or is it just really common in human health to find chemicals with a therapeutic range around the one millimole per liter level?
If lots of chemicals have a therapeutic range around one millimole, then the intersection of therapeutic ketone and therapeutic lithium levels would be less surprising.
If few chemicals had a therapeutic range around that region, then it would be more surprising to see that intersection occur, and it would then be worth probing whether that is a clue to some biological mechanism. (Perhaps that is a common saturation level where enough neurons are exposed to the chemicals to prevent any breakout manic wildfires?)
To answer this question, we need to know how likely it is that 2 randomly selected blood chemicals would have overlapping therapeutic ranges.
How many blood tests are there?
To know the odds that any two random blood tests would have an overlapping target range, we first need to know how many different blood chemistry tests there are.
Just how many chemicals are there? According to the WHO, humans have now labeled over 160,000,000 chemicals! If you measured your blood levels for each one of these chemicals with one drop of blood per test, doing one test a second, you would be dead by tomorrow night and only have done 00.00075% of the tests.
So, let's narrow our list of chemicals to just the ones commonly found in human blood.
An example besides ketones and lithium is glucose. For glucose, the normal blood level range is between 3.9 and 6.1 mmol/L. Levels below 3.9 mmol/L start to be considered hypoglycemic, with levels below 2.8 mmol/L can be dangerous. Levels above 7.8 mmol/L when fasting start to be considered hyperglycemic, or above above 11.1 mmol/L 2 hours after a meal.
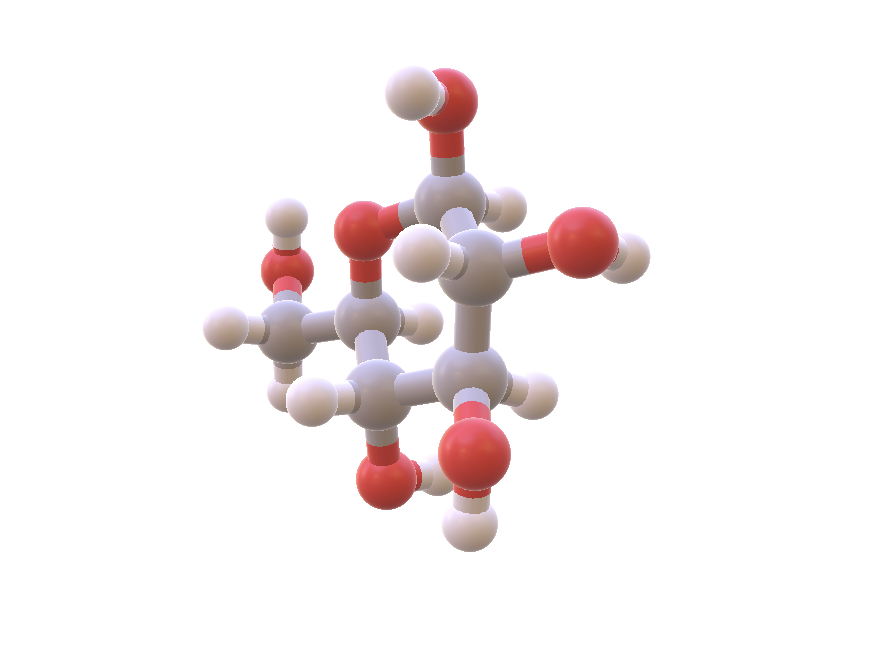
Glucose, C_6H_{12}O_6, is another tiny molecule. A carbohydrate, it is the primary fuel of metabolism. Glucose blood tests can easily be done at home. Image Source: NIH
My first day looking for a good dataset of chemicals found in human blood with reference ranges did not go well. I spent a couple of hours without success. So I started making my own.
On day 2, while continuing to build my own blood test dataset for this post, I found a page on Wikipedia that already has a list of the most common blood tests with reference ranges, and some Wikipedians had even made a visualization already. After cursing myself for missing this on the first day, I switched to being grateful to the Wikipedians and started enhancing my own dataset with this information. I also found this page on Wikipedia that lists 240 human blood components.
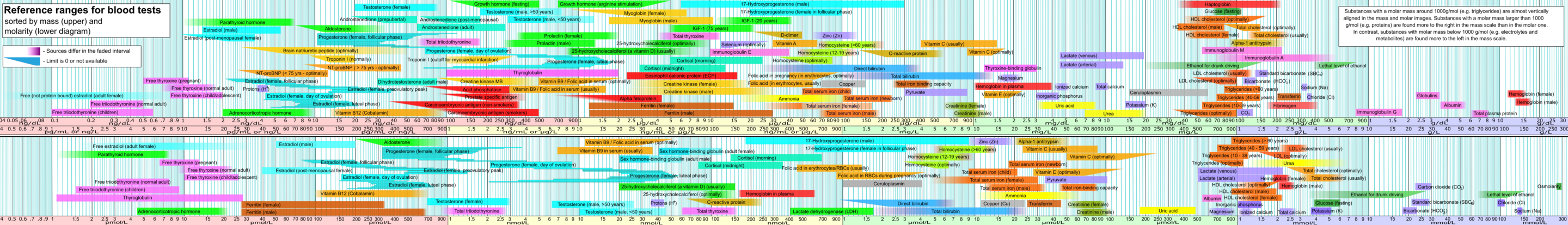
I spent a couple of hours building my own dataset to make a chart like this, then found out it's already been done on Wikipedia.
I was thrilled to find this information on Wikipedia, but still it seemed like a small number of chemicals. Are none of the other 160,000,000 chemicals relevant? I kept searching and finally found a team who built an amazing website called HMDB that lists 3,126 chemicals that would be relevant to my question. I felt good knowing that I could do this initial analysis using the Wikipedia data, and if it seems worthwhile in the future, the HMDB data can be used to do it again at a 10x scale.
Finally I had enough raw material to start building my first structured, clean, tabular dataset to answer my question. It was a bit of data cleaning grunt work, and v1 is pretty ugly and I'm sure is packed with loads of errors, but finally I had a dataset of the reference levels for around 240 chemicals found in human blood.
So now I can finally answer: one millimole, what are the odds?
My Answer (v1): 1 in 20
The reference levels of only 14 of the 241 chemicals in my dataset have an overlap with the therapeutic ranges of ketones and lithium. The only other chemicals that have overlap with these levels are listed below:
- Urea
- Glucose
- Potassium
- Creatine Kinase
- Lactate Dehydrogenase (LDH)
- Calcium
- Triglycerides
- Alkaline Phosphatase
- HDL Cholesterol
- Phosphate
- Magnesium
- Albumin
- Cortisol
- Uric Acid
If someone called me at this moment, and offered me even odds as to whether it was just a random coincidence that there is overlap in the therapeutic ketone range and the therapeutic lithium range, or whether it was because of some related biomechanisms I would bet heavily that it is not a coincidence. This would be great, because I would be able to honestly say I did my best in the time allotted, made the best decision with the information I had, and could stop work on this frighteningly long blog post.
Alas, the phone did not ring. So I can't "call it a day" just yet.
Especially because I got an answer that makes me look good. This is often a sign of wishful thinking. Imagine if it turned out that half of all chemicals in the blood had target levels around 1 mmol/L, and that this was a commonly known fact to everyone in medicine and chemistry. If I found that answer, then I might feel silly for spending so much time on such a naive hunch. The fact that my hunch seems more interesting now, makes me think I may have biased my dataset.
I must walk around my work and do another inspection.
The biggest flaw in my current research is that I haven't made an extra effort to include other chemicals taken by bipolars in my dataset. Let's do that now.
I went back and added 8 drugs by hand to my dataset including the common bipolar medications Valproate, Lamictal, Zyprexa, and Abilify. Of the 8, only one has a bit of overlap: Valproate (~.2 - .5). Interestingly enough, the bipolar medication that usually ranks #2 (after lithium), is...Valproate!
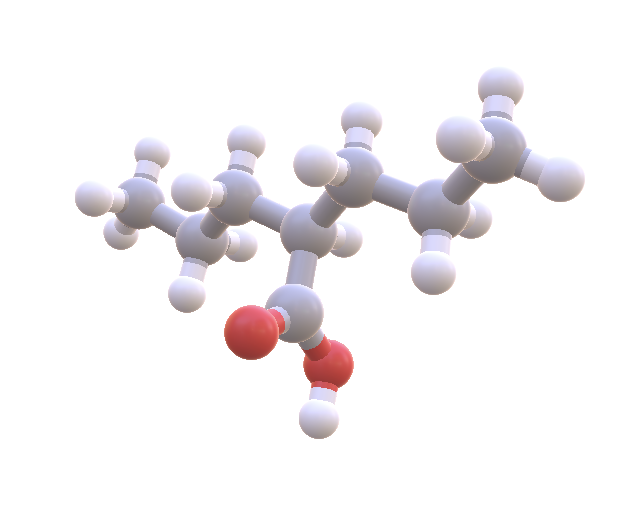
Valproate (aka Depakote) is often ranked 2nd among bipolar medications and also has overlap with the ketone/lithium range. It also crosses the blood brain barrier by passive diffusion. Image Source: NIH
So I expanded my dataset yet still found it unlikely that 2 random chemicals would have therapeutic level overlaps. At this point, I am confident concluding this leg of my research journey. With this additional data I would not change my initial bet. My answer is: the slight overlap in the therapeutic blood level ranges of the 2 top bipolar drugs with the therapeutic level of ketones is a strong clue to some underlying biomechanisms.
My research won't stop here. But this blog post will.
This post is already maybe my longest, and if it gets much longer not even the author would want to read it.
Also, given that my background in chemistry consists of the past 1 week of Internet searches combined with 1 year of high school chemistry 22 years ago, there could be some really naive, glaring mistakes in this post, such as flaws in my mental models or huge errors in my datasets (largely assembled with copy/paste and LLMs), that radically alter the conclusions. It's better that I publish what I have now, and maybe a reader could alert me to suboptimal models or paths I've followed.
This post may not describe my future understanding, but does describe my current understanding, and my journey getting here.
I am looking forward to researching the next logical question: what might those common biomechanisms be? How might ketones and lithium work in bipolar? Perhaps there will be a Part II to this post.
But for now, if you'll excuse me, I've got fats to eat, and fingers to prick.
Notes
[1] There is a dangerous condition involving ketones called ketoacidosis affecting mostly diabetics which is associated with around 200,000 cases and 600 fatalities per year in the US.
[2] A good starting place to start reading about some of the keto for bipolar pioneers is Metabolic Mind.
[3] I should note that there is recent research showing that astrocytes (a kind of brain cell) may also produce ketones. I did not dive deep down that thread yet, but thought I should mention it since it involves ketones and the brain, which is ultimately what I'm interested in.
[4] I am using the one millimole number to simplify the writing a bit, but when you visualize things keep in mind we're thinking about overlapping ranges rather than a specific point.
Thanks to RGM for feedback on this post.